According to Johns Hopkins researchers Debes et al. (2021), Covid-19 vaccines followed by little to no side effects still produce an adequate amount of spike antibodies to neutralize SARS-Cov-2. Pfizer-BionTech clinical trial results showed that 50% of participants did not report significant side-effects, yet 90% developed immunity against Covid-19. Moderna also reported that only 10% of its clinical trial participants experienced common side effects, but 95% still developed immunity. Different people show different reactions when developing protective immunity against viruses. In most cases, the innate immune response is what causes common side-effects by initiating inflammation, while the adaptive immune response only assists innate immunity throughout the process (Cronkite & Strutt, 2018). When the inflammatory response is exaggerated in some people, side-effects such as injection site pain, fever, fatigue, diarrhea, and dizziness may occur. Previous studies suggest that several factors such as age and gender may contribute to the exaggeration of inflammatory immune responses. People above the age of 65 have been reported to show fewer side-effects to the Covid-19 due to gradual age-related decline in immune activity (Weinberger et al., 2008). This decline is also related to lower antibody levels, but adequate protection against the virus is still provided. Women are almost 4 times more likely than men to show vaccine side-effects (79% to 31%) (Gee et al., 2021). Since testosterone is known to help suppress inflammation, men who produce 20 times more testosterone than women experience fewer side-effects (Kanda et al., 2003). However, antibody count is not affected.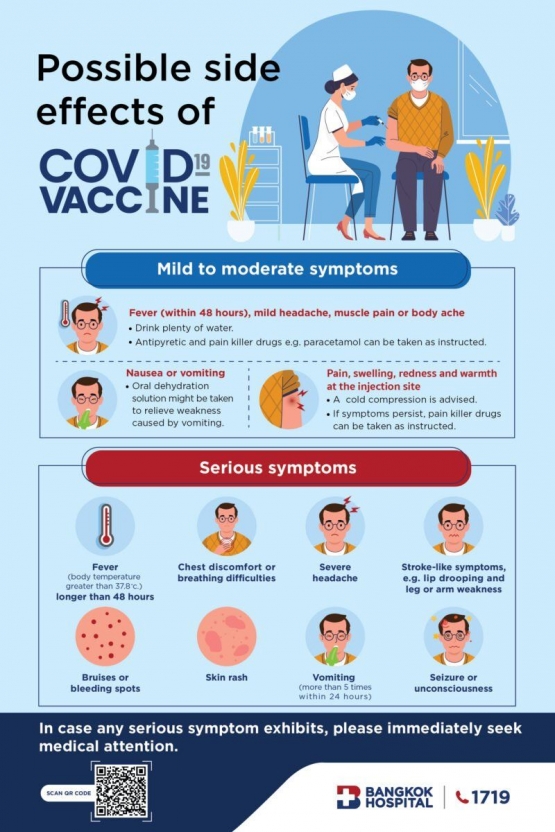
Conclusion
Although different people show distinct reactions to the Covid-19 vaccine, immunity against Covid-19 is still established. Either way, the vaccine provides protection. Therefore, all WHO-approved Covid-19 vaccines are effective in protecting the body against serious Covid-19 illnesses. Vaccine brands and side effects should not be the basis of deciding whether or not to get vaccinated. You too, should get vaccinated immediately (if not already)--- to protect yourself and your loved ones.
Click here to register yourself for a Covid-19 vaccine in Indonesia!
References
- Beattie, S. (2021). He's had 3 COVID-19 shots. So why does he have to isolate before seeing his Canadian grandkids? CBC News.
- Bloom, J. D., Chan, Y. A., Baric, R. S., Bjorkman, P. J., Cobey, S., Deverman, B. E., Fisman, D. N., Gupta, R., Iwasaki, A., Lipsitch, M., Medzhitov, R., Neher, R. A., Nielsen, R., Patterson, N., Stearns, T., van Nimwegen, E., Worobey, M., & Relman, D. A. (2021). Investigate the origins of COVID-19. Science, 372(6543), 694--694. https://doi.org/10.1126/science.abj0016
- Callaway, E. (2022). Why does the Omicron sub-variant spread faster than the original? Nature, 602(7898), 556--557. https://doi.org/10.1038/d41586-022-00471-2
- Cronkite, D. A., & Strutt, T. M. (2018). The Regulation of Inflammation by Innate and Adaptive Lymphocytes. Journal of Immunology Research, 2018, 1--14. https://doi.org/10.1155/2018/1467538
- Debes, A. K., Xiao, S., Colantuoni, E., Egbert, E. R., Caturegli, P., Gadala, A., & Milstone, A. M. (2021). Association of Vaccine Type and Prior SARS-CoV-2 Infection With Symptoms and Antibody Measurements Following Vaccination Among Health Care Workers. JAMA Internal Medicine, 181(12), 1660. https://doi.org/10.1001/jamainternmed.2021.4580
- Fauzia, M. (2021). Kenapa pemerintah memilih vaksin Covid-19 Sinovac? Ini 3 alasannya. Kontan News.
- Gee, J., Marquez, P., Su, J., Calvert, G. M., Liu, R., Myers, T., Nair, N., Martin, S., Clark, T., Markowitz, L., Lindsey, N., Zhang, B., Licata, C., Jazwa, A., Sotir, M., & Shimabukuro, T. (2021). First Month of COVID-19 Vaccine Safety Monitoring --- United States, December 14, 2020--January 13, 2021. MMWR. Morbidity and Mortality Weekly Report, 70(8), 283--288. https://doi.org/10.15585/mmwr.mm7008e3
- Liddell, K., Skopek, J. M., Palmer, S., Martin, S., Anderson, J., & Sagar, A. (2020). Who gets the ventilator? Important legal rights in a pandemic. Journal of Medical Ethics, 46(7), 421--426. https://doi.org/10.1136/medethics-2020-106332
- Mallapaty, S. (2021). WHO approval of Chinese CoronaVac COVID vaccine will be crucial to curbing pandemic. Nature, 594(7862), 161--162. https://doi.org/10.1038/d41586-021-01497-8
- National Center for Immunization and Respiratory Diseases. (2022). Understanding Viral Vector COVID-19 Vaccines. Centers for Disease Control and Prevention.
- Nugraheny, D. E. (2020). 6 Alasan Pemerintah Mengapa Beli Vaksin Covid-19 dari Sinovac China Artikel ini telah tayang di Kompas.com dengan judul "6 Alasan Pemerintah Mengapa Beli Vaksin Covid-19 dari Sinovac China. Kompas.
- Our World in Data. (2022). Covid-19 Cases Overview. Our World in Data.
- Ranney, M. L., Griffeth, V., & Jha, A. K. (2020). Critical Supply Shortages --- The Need for Ventilators and Personal Protective Equipment during the Covid-19 Pandemic. New England Journal of Medicine, 382(18), e41. https://doi.org/10.1056/NEJMp2006141
- Rogin, J. (2021). The United States can't ignore China's vaccine diplomacy in Latin America. The Washington Post.
- Singaporean Ministry of Health. (2022). COVID-19 case numbers.
- Washington State Department of Health. (2022). COVID-19 Cases, Hospitalizations, and Deaths by Vaccination Status.
- Weinberger, B., HerndlerBrandstetter, D., Schwanninger, A., Weiskopf, D., & GrubeckLoebenstein, B. (2008). Biology of Immune Responses to Vaccines in Elderly Persons. Clinical Infectious Diseases, 46(7), 1078--1084. https://doi.org/10.1086/529197
- World Health Organization. (2021). The Sinovac-CoronaVac COVID-19 vaccine: What you need to know. World Health Organization.
- Yiu, P. (2021). Sinovac booster insufficient against omicron, study shows. Nikkei Asia.
- Zhang, Y., Zeng, G., Pan, H., Li, C., Hu, Y., Chu, K., Han, W., Chen, Z., Tang, R., Yin, W., Chen, X., Hu, Y., Liu, X., Jiang, C., Li, J., Yang, M., Song, Y., Wang, X., Gao, Q., & Zhu, F. (2021). Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18--59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. The Lancet Infectious Diseases, 21(2), 181--192. https://doi.org/10.1016/S1473-3099(20)30843-4
Baca konten-konten menarik Kompasiana langsung dari smartphone kamu. Follow channel WhatsApp Kompasiana sekarang di sini: https://whatsapp.com/channel/0029VaYjYaL4Spk7WflFYJ2H















