Viral vector vaccines for Covid-19 such as Sinovac and Johnson and Johnson have been approved by the WHO, CDC, and FDA after surpassing safety and efficacy standards. Thus, it is safe to receive viral vector vaccines (National Center for Immunization and Respiratory Diseases, 2022).
Although no vaccine is 100% protective, all of the approved vaccines will provide protection against serious illness, hospitalization, and death. The WHO states that whatever vaccine is made available should be immediately taken to provide immunity. Available vaccines in a certain country depends on national authorization or diplomatic relationships. China-produced vaccines such as Sinovac have been proved to be 51% effective in preventing Covid-19 in late-stage trials in Brazil, and trials in Indonesia and Turkey showed a higher efficacy rate of up to 84%. Based on a post-trial study conducted in Chile, Sinovac was also 80% effective at preventing death from Covid-19 despite the emergence of new variants (Mallapaty, 2021). Beijing-produced Sinopharm was also proved to be 79% efficacious against symptomatic Covid-19 (Mallapaty, 2021).Respiratory Diseases, 2022).
Despite surpassing the safety and efficacy standard upheld by the WHO, Sinovac and Sinopharm are mostly only administered in low-income countries such as Chile and Bostwana. On the contrary, high-income countries in America and Europe neither authorize nor recognize China-produced vaccines, especially since these countries have raised concerns regarding the Wuhan lab-leak theory. Then-president Donald Trump of the United States was the first to promote this controversy in 2020, and current President Biden called upon a global investigation to consider this possibility in 2021. WHO-appointed scientists Bloom et al. (2021) directly investigated this case and concluded that the lab-leak theory was extremely unlikely, but Dr. Anthony Fauci, Chief Medical Adviser of the United States, continued to express concerns regarding this issue. China immediately denied accusations of engineering SARS-CoV-2 as a bioweapon, and even threatened the U.S. Government that it would withhold crucial supplies such as vaccines if the current administration continued to accuse China of mishandling the outbreak. Following this threat, the Biden Administration refused to accept the heavy-handed vaccine diplomacy from China, stating that the Chinese Communist Party was pushing its economic expansion and political agenda in the Western Hemisphere (Rogin, 2021). Other countries have since done the same, but with less public reasons.
Another American country, Canada, currently approves only 6 out of 9 WHO-approved vaccines, namely Moderna, Pfizer-BionTech, AstraZeneca, Johnson and Johnson, Novaxovid, and Medicago Covifenz. Jillian Kohler, Director of the WHO Collaborating Centre for Governance, Accountability, and Transparency in Pharmaceutics states that Health Canada has not yet approved of Sinovac and Sinopharm due to transparency issues. Sinovac and Sinopharm are both produced by Chinese vaccine manufacturers who do not provide "reliable and transparent" clinical trials as compared to vaccines produced in Western countries (Beattie, 2021). Countries in the European Union also do not approve of Chinese-manufactured vaccines for the same transparency issues. The EU vaccine passport only accepts American and European-produced vaccines including Moderna, Pfizer-BionTech, AstraZeneca, and Johnson and Johnson, meaning travelers are only allowed entry into specific countries based on the brand of the vaccine they received. Even AstraZeneca-Oxford, which is originally manufactured in the United Kingdom, will not be accepted if the vaccine was manufactured under the license of the Serum Institute in India. On the contrary, China has not yet approved of foreign vaccines including Pfizer-BionTech and Moderna, directing their people to receive self-produced Sinovac and Sinopharm instead.
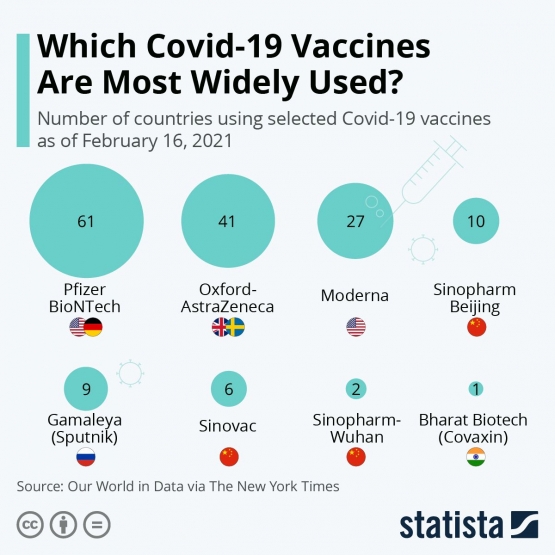
Interestingly, Sinovac is the most sought-after vaccine brand in Indonesia. Based on data provided by the National Ministry of Health (2020), 186 out of 235.6 million doses (79%) administered between January and October 2021 were Sinovac vaccines. According to Health Minister Budi Gunadi Sadikin, the Indonesian government had only 3 major considerations, namely approval, authorization, and stock before choosing Sinovac as the main Covid-19 vaccine for its people. Sinovac was the first batch of vaccines to arrive in Indonesia and had already been approved by the WHO as well as authorized National Food and Drug Supervisory Agency (BPOM) for immediate administration. Indonesia had also purchased Novavax, Pfizer-BionTech, and AstraZeneca-Oxford vaccines, but all of them could not be delivered on time and had to be further evaluated by BPOM before receiving national authorization (Fauzia, 2021). Following the advice given by WHO for local authorities and unvaccinated people, the Indonesia government provided whatever vaccine was available first, advising citizens that "the best vaccine is the one available for grab". Siti Nadian Tarmizi, a Covid-19 vaccine spokesperson for the National Ministry of Health also added several more points. Tamizi stated that dosage count, affordability, and product supply to distribution channel were also important deciding factors. As an affordable single-dose vaccine, Sinovac was considered an accessible and efficient vaccine brand. Furthermore, Sinovac vaccines could be locally manufactured by a state-run pharmaceutical company, BioFarma, under license from the main Chinese company (Nugraheny, 2020). Local production meant less delivery time and easier distribution, both of which benefit the vaccination process in Indonesia. Other countries should follow in the footsteps of nations like Indonesia who authorize all WHO-approved vaccines to prioritize herd immunity as a strategy to curb the pandemic.

Why Are Booster Brands Different?
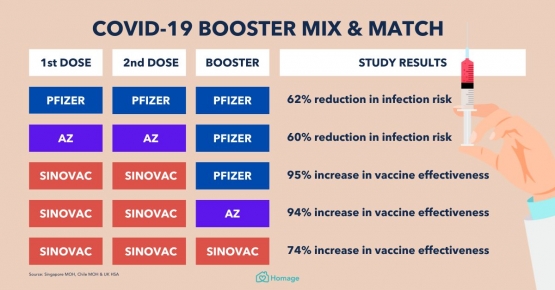
With the ever-evolving mutation of viruses, including SAR-CoV-2, lethal variants such as delta in late-2020 and omicron in late-2021 have emerged to create a spike in infection, hospitalization, and death rates. The WHO, CDC, and central governments have since urged all eligible individuals (those aged 18 and above who have received 2 prior vaccination shots) to receive an additional booster shot. Since most countries in America and Europe do not recognize Sinovac due to transparency issues, it is only reasonable that the same brand is not recognized as a booster in the same countries. However, in Indonesia where nearly 80% of the citizens had already received Sinovac vaccines, Sinovac boosters are not authorized. Instead, only Pfizer-BionTech and Moderna vaccines are provided as boosters by the government. According to Sinovac, 94% of trial participants who had received 3 Sinovac shoots produced a sufficient amount of neutralizing antibodies. However, results from a study conducted in Hong Kong showed that Sinovac booster shots do not provide adequate protection against the omicron variant, while Pfizer-BionTech boosters significantly improved protection for those who had previously received 2 Sinovac shots (Yiu, 2021). These results are reasonable as omicron was reported to be 10 times better than delta at evading Covid-19 vaccines, meaning that not all of the vaccines are effective against certain variants (Yiu, 2021). Nevertheless, Sinovac vaccines are still effective when taken as the first two shots, and should be taken if available.
Are No Side Effects A Bad Sign?
As a 17-year-old Indonesian citizen, I enthusiastically received the full dosage of Sinovac vaccine (0.25 mL per dose) in mid- to late-2021. During both vaccinations, I did not feel any side effects except for a sore arm and drowsiness for a few hours. After consulting 3 of my family members (female aged 19, female aged 48, and male aged 49) who had also been vaccinated with Sinovac, they reported the same side effects. All 3 of them also received a Moderna booster (0.5 mL) 6 months later. Two members reported feeling fatigued, dizzy, and feverish at night, while the other only reported diarrhea. Meanwhile, an Australian relative (male aged 30) who had gotten the Moderna shot and booster reported severe stomach pains, diarrhea, fatigue, and a high fever that lasted for 2-3 days. Based on phase 1 and 2 clinical trial data published in The Lancet, participants who received Moderna and AstraZeneca-Oxford vaccine shots reported a higher occurrence of fever (Zhang et al., 2021). Other common infects include fatigue, diarrhea, and injection-site pain and muscle pain (13-21%).
When comparing the side effects of regular (first and second dose) to booster shots, it is important to note that regular vaccines are administered twice with a dosage of 0.25 mL each time over the span of a month, while booster shots are given all at once with a full dose of 0.5 mL. It is only reasonable that a higher dose leads to more significant side effects, but why do people who receive the same shot from the same brand (e.g. Moderna booster) still experience different reactions? Previous studies show that fevers following a vaccination act as thermal regulators that ensure the immune system will respond to the vaccine (Evans, 2015). Thus, the observation of distinct side effects between vaccine brands led to the following question: does the absence of side effects following a vaccination indicate a low antibody response?
















