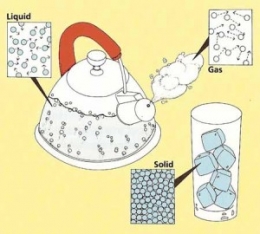
Atoms make up everything we know from your chair to your bicycle. But not all atoms are the same. Believe it or not, atoms have different behaviors. Some are crazy and wild and go all over the place. Others tend to be quiet and stay put.
It is very normal to see these things happen. You see the behaviors of these atoms in daily basis. Water is a great example. Water is a liquid. And the reason why liquids tend to spread everywhere when you spill your glass of water on the table is because of the atoms.
[caption id="attachment_218899" align="aligncenter" width="300" caption="Water spilling (Doc: einxh.tistory.com)"][/caption]
The H2O molecules in a liquid are always moving around, bumping here and there. They are constantly moving. Imagine a crowded area in the city, people are trying to go to their own destinations and are bumping in to each other.
You can also imagine that all the atoms in the water are dancing crazily in a small room. So when the glass spills all of the water and it ends up on the table, the dancing atoms get more room to dance so they spread out. It’s kind of a funny way to explain it.
Solid matter is like, for instance a rock. Atoms in a rock are like random people that don’t know each other, sitting in a room full of other atoms. There is no space to move. And that is why it is called solid or united.
This is the reason why rocks are different than water. Water atoms are crazy while rock atoms are quiet. But there is a way to change the characteristics of an atom. You can change a quiet atom into a wild on and it’s quite easy.
Ice is solid matter, its atoms are still. They’re not moving. But if you microwave or just leave the ice out in sunlight, the atoms start to get crazy and spread apart. And if you freeze ice, which is the other way around, the atoms change from being wild to neat.
[caption id="attachment_218897" align="aligncenter" width="300" caption="Water Molecules (Doc: daviddarling.info)"]
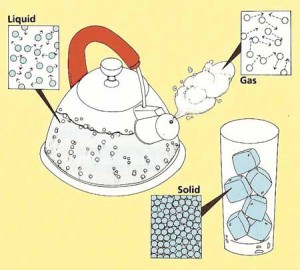
Gas is also a substance in the universe, and it is way freer than water. They rapidly move away from each other when uncontained. Smoke is an example. When firewood is burned, the smoke rises up, then spreads all over the atmosphere.
Metals are awesome right? Not the metal music. But the actually metal for instance, aluminum, tin, iron. Steel is a compound (made of more than 2 types of atom) made mostly from iron, but parts of it contain carbon. Coal is high in carbon so that’s why they mix them up together.
Metals are shiny and most of them are naturally solid. But in fact, there are some metals that are naturally liquid. Have you ever heard of Mercury? Mercury is the only metal that is liquid in room temperature. Room temperature is about 20 degrees Celsius, not too hot or cold or, the ideal temperature.
[caption id="attachment_218900" align="aligncenter" width="300" caption="Pouring Liquid Mercury (Doc: en.wikipedia.org)"]

All the metals can melt and turn into a liquid at a certain heat. But Mercury tends to fascinate me. I like things that stand out from the others, and mercury does just that. Thanks for reading!
Baca konten-konten menarik Kompasiana langsung dari smartphone kamu. Follow channel WhatsApp Kompasiana sekarang di sini: https://whatsapp.com/channel/0029VaYjYaL4Spk7WflFYJ2H