Introduction
Generally polymerization divided two methods. They are step growth polymerization and step growth polymerization. We can see detail branching of polymerization in diagram below.
In this experiment, we can investigate about anionic polymerization to make copolymerization via block copolymers and homopolymer. Anionic polymerization occurs when negative active specie attacks the monomer. We used Buthyl lithium as initiator in this experiment (we can see the structure in figure 3). To make copolymer of Polystyrene and PMMA (poly Methyl methacrylate) we need 1,1 diphenyl ethylene (figure1)as capping agent. Dimethoxyethane (DME) as polar solvent was used for buthyl lithium in this reaction.
There are some types of copolymers which they are homopolymer, alternating copolymer, random copolymer, block copolymer and graft copolymer. In this case we will make block copolymer (figure2) and homopolymer. In block copolymers will be made by PS-b-PMMA via anionic polymerization.
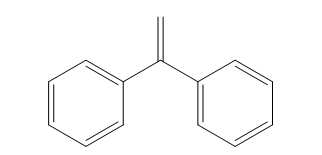
Figure1. Structure of 1,1 Diphhenyl ethylene
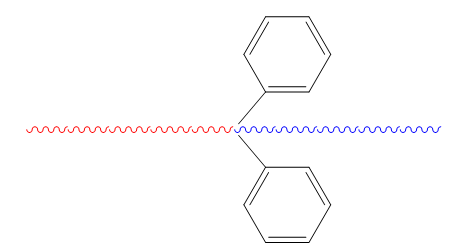
Figure2. Structure of Block copolymers (Polystrene(Red)-PMMA(blue))
Reaction Mechanism
In this experiment we have some chemicals which were used. Buthyl lithium as initiator, styrene and methyl methacrylate as monomers, some solvent were used such as DME (dimethoxyethane), and toluene. 1,1 diphenyl ethylene as capping agent. In the end process of polymerization for quenching, we use trimethylsilyl chloride. For detail process of anionic polymerization from beginning to last process, we can see figure 3 until figure 10.
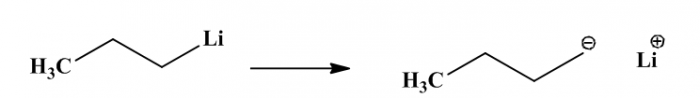
Figure3. Buthyl lithium(BuLi) as initiator
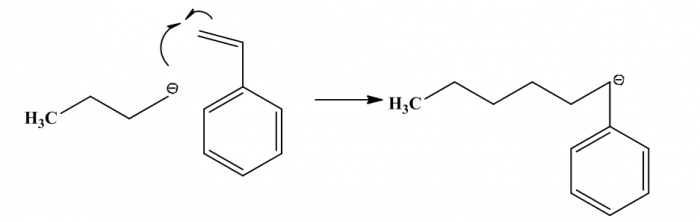
Figure4. Initiation of styrene polymerization
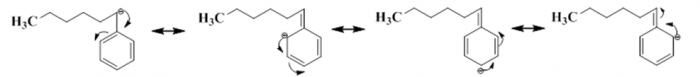
Figure5. Resonance effect of anionic polymerization of Styrene
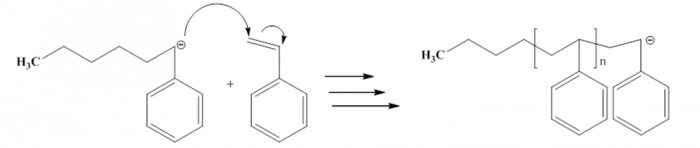
Figure6. Propagation process of Polystyrene
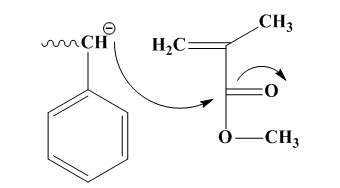
Figure7. Block copolymerization process of polystyrene and polymethylmethacrylate
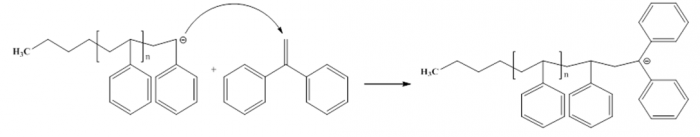
Figure8. Additional process of 1,1 diphenyl ethylene
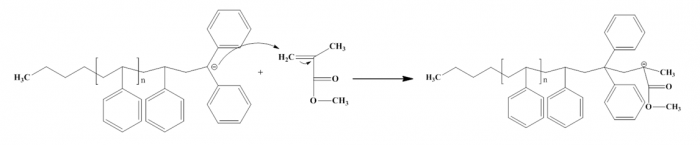
Figure9. Propagation of copolymerization polystyrene and PMMA
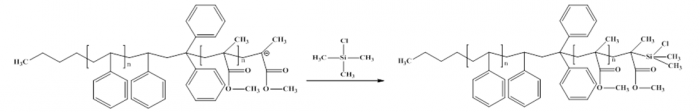
Figure10. Quenching /Termination of copolymerization polystyrene and PMMA by trimethylsilyl chloride
Experiment
Synthesis of anionic block copolymerization of styrene and Methyl methacrylate (MMA) will become the structure like P(buli-polystyrene-diphenyl ethylene-PMMA). In this experiment we used DME as polar solven for Buthyl lithium (BuLi). In this experiment we also use diphenyl ethylene because we wanted to make block copolymers with it as capping agent.
The second part of this experiment, we made homopolymers of styrene by diphenylethylene as capping agent in their structure. The chemical composition in their structures (PS-PMMA and homopolymer of PS) can be known by H-NMR analysis (figure 11 and figure 12). In figure 11, we can determine the chemical composition which we can see the different frequency of monomer structure and polymer structure. In this picture (figure 11 and figure 12), we have to know the difference between the thin graph and the bigger graph. The bigger one (bigger area graphic) is analysis of polymers and the thin graph with higher graph is monomer structure.
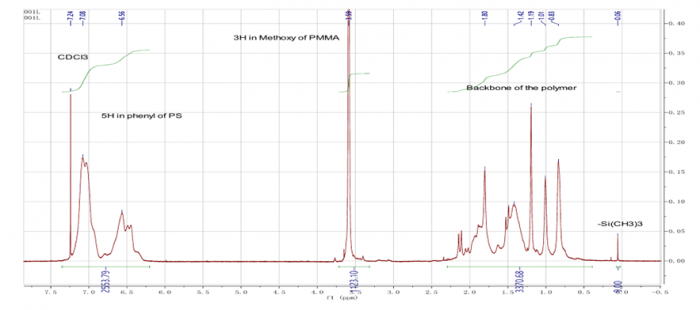
Figure11. H-NMR analysis of Polystyrene-b-PMMA
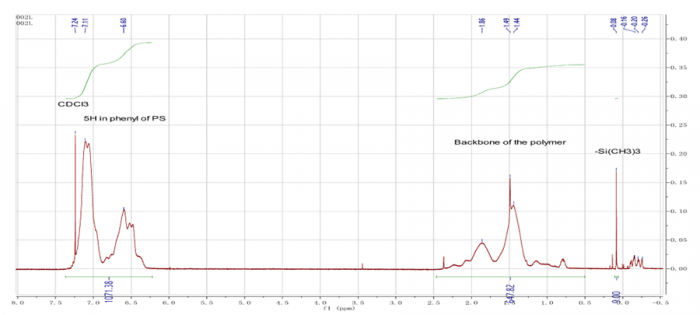
Figure12. H-NMR analysis of Polystyrene
Follow Instagram @kompasianacom juga Tiktok @kompasiana biar nggak ketinggalan event seru komunitas dan tips dapat cuan dari Kompasiana. Baca juga cerita inspiratif langsung dari smartphone kamu dengan bergabung di WhatsApp Channel Kompasiana di SINI




















