Differential Scanning Calorimetry (DSC) is one technique for knowing or analysis Heat Capacity. On other hand by analysis DSC we can know glass transition, crystallization behavior, melting temperature of material which DSC is really important for knowing how to treatment position of material by temperature control. So, we can know the technique for using DSC equipment and DSC analysis. In Industries, this technique is really important to control material shaping and sizing by temperature and heat flow.
The principle of the technique and the theoretical basis
The thermal analysis has some different techniques to measure physical sample properties such as a function of temperature, which the sample is observed to treat heating or cooling. In differential scanning calorimetry(DSC) depends on differences in energy required between the sample and the reference at same temperature. On the other words DSC is thermal measurement to analysis transition enthalpies in the samples at heating rate [1]. The glass transition, crystallization, melting temperature, heat capacity and degree of crystallinity can be determined by this measurement in figure 1.
The important parameters to know in DSC measurement that is Heat Capacity (Cp). The heat capacity of the system is the quantity of heat needed to raise the temperature of the system of 1oC. To obtain the heat capacity we have to know the equation (1).
Figure1. The entire DSC Plot of glass transition, crystallization, and melting temperature
The thermograms of DSC Plotting give some information about the transitions that polymers pass through in the temperature range of interest. Glass transition is shown as step change. Melting temperature is represented by up peak. The area under the melting curve is proportional to amount of heat required to melt the sample such as the heat of fusion. If we know the theoretical heat of fusion of pure polymer, we can calculate the degree of crystallinity of the sample from its measurement by equation (2) [2].
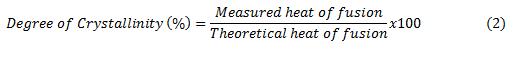
The measurement for thermal transition in materials uses the technique of traditional Differential Scanning Calorimetry (DSC). There is some information to know temperature transition occur. Modulate DSC is an extension of DSC for measuring a sinusoidal temperature oscillation (modulation) on the linear temperature ramp. The capabilities of traditional DSC need to extend via the modulate DSC. Generally problems of DSC measurement have three general categories such as analysis of complex transition, Sensitivity and Increasing resolution. The general equation is obtained for describing calorimetric response mathematically by equation (3) [3].
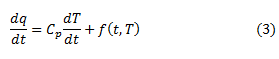
Experimental Part
In the first section of this experiment we have to prepare sample of PET to determine glass transition, melting and crystallization temperature by DSC curve. Thus we have to compare two results of the sample by following the step below:
Preparing sample of PET:
1.Cut a piece of PET film from the plastic bottle, clean with water and dry it.
2.Make the film of PET with the weight 7.5 mg
Then the second step of this experiment we have to prepare the granule of PS sample after that making the granule become powder then weight PS 6.6 mg. In this section we have to determine weak glass transition and heat capacity data.
Both of the samples after preparation we have to encapsulate each samples into aluminum pans then press the samples and references (empty sample) of each aluminum pans inside the presser. After that the sample and the references have to put them into DSC machine for measurement.
In the first section, we have to set up the software of DSC measurement with some parameter control. The parameters that will program the DSC to start from equilibrium state of 50°C, ramping 5°C per minute all the way to 300°C. Nitrogen was chosen as Gas 1, with a flux of 35 ml/min, and there was no Gas 2.
In the second section, we have to set up modulate DSC in software measurement with some parameter: The equilibrium state is at 60oC, modulate +/- 0.531o C every 40 seconds, Isothermal is set up to 5 min, and Ramp 5oC to 200oC.
Result and Discussion
Thermal behavior of PET
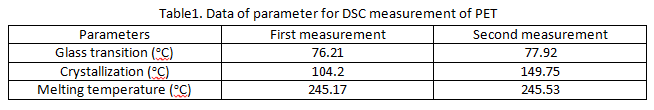
The measurement of DSC for PET we got two results in figure 2 and figure 3 which we collect the data in table 1. The comparison data in table1 that there is really difference value of crystallization. In the first measurement, we got value is 104.2oC and the second measurement value is 149.75oC. Theoretically there is no difference value of crystallization because we still use same material with same behavior. Unfortunately we obtain the value differences. Maybe the reason that can be different because two reasons, they are the first is error of temperature reader of machine and the second is molecules of PET got impurities of air. The air can go into aluminum pans because the aluminum pans are not closed perfectly when getting high temperature on the aluminum pans.
We compared the value of glass transition, crystallization and melting temperature in some literature that the value of glass transition is 80oC, melting temperature around 250oC - 255oC and crystallization temperature is 140oC. So we can get the conclusion from this section that approximately values of the second section are almost same with literature values.

Figure2. The first DSC measurement of PET
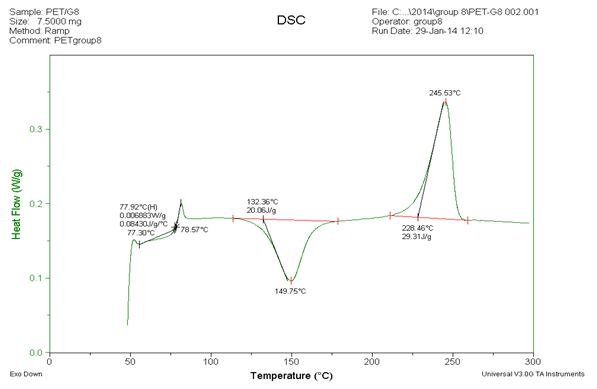
Figure3. The second DSC measurement of PET
Determine the heat capacity of PS and weak glass transition
The second experiment uses PS as sample that represents in figure 4 and figure 5 that we can obtain the value of glass transition of PS is 98.22oC at reversing heat flow curve in figure4. According to equation 3
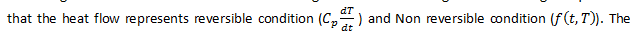
reversible condition has correlation with glass transition and melting temperature. So we can obtain glass transition value in reversing heat flow curve.
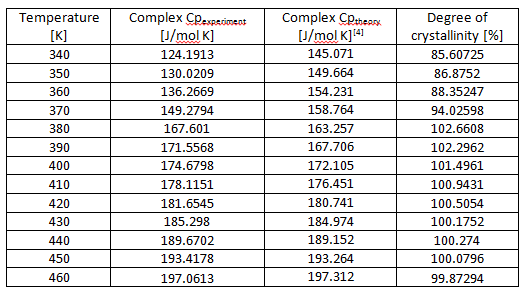
In another analysis about heat capacity data, we can see in figure 5 and table 2. Based on the value of glass transition is 98.22oC (371.37 K). Thus we can obtain the value of heat capacity from table 2 is 149.8949 J/mol K by extrapolation. In table 2 we can see that the value of heat capacity by experiment and by theoretically which both of the value we can obtain degree of crystallinity by equation (2). Degree of crystallinity has function to know the different between “crystalline” and “amorphous”. According to table 2 that we know the values of degree of crystallinity of PS around 97.167 % that means PS is crystalline polymer. So that why the curve of glass transition almost discontinue.
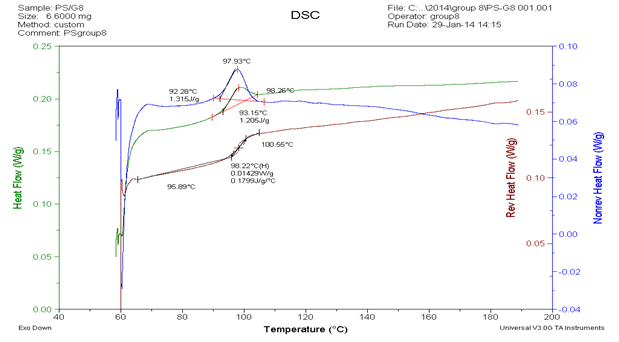
Figure4. The weak glass transition curve analysis of PS
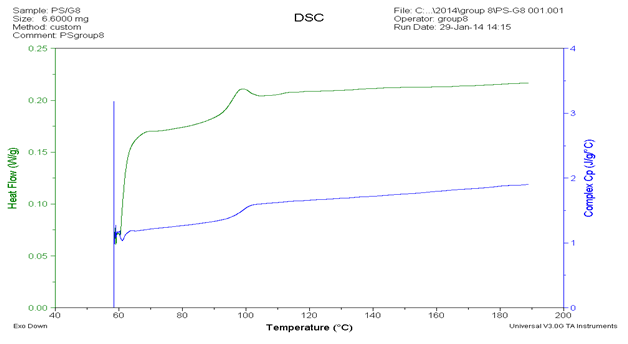
Figure5. The heat capacity curve analysis of PS
Table2. The comparison data of heat capacity measured and heat capacity theoretical of PS
References
[1] Ellipsometry and polarized light, R.M.A. Azzam and N.M. Bashara, Elsevier Science, Amsterdam, 1987
[2] Optics (3rd edition), E. Hecht, Addison Wesley Longman, Reading, 1998
[3] http://homepages.rpi.edu/~plawsky/Research/images/Spinning.jpg
[4] http://www.mathsisfun.com/numbers/complex-number-calculator.html#functions
[5] http://refractiveindex.info/?group=CRYSTALS&material=SiO2
This is a little bit information and technique for all Indonesian people. I hope this information can be useful for us. I am sorry that I wrote my article in English because I think English is very important language now. If you want detail about other technique related to polymer science and chemical engineering you can ask me at hardiyantoputra@gmail.com or www.facebook.com/hardiyanto.wijaya . You can use Bahasa Indonesia, Jawanese or English what you want to.
Dan jika Anda berkenan untuk mengetahui detail berbagai informasi seputar kehidupan sebelum bisa belajar ke Jerman dan selama hidup di Jerman dalam dua Bahasa. Semua apapun yang sudah saya pelajari dan tulis silahkan mengunjungi http://hardiyantoputra.wordpress.com/
Follow Instagram @kompasianacom juga Tiktok @kompasiana biar nggak ketinggalan event seru komunitas dan tips dapat cuan dari Kompasiana. Baca juga cerita inspiratif langsung dari smartphone kamu dengan bergabung di WhatsApp Channel Kompasiana di SINI
















