Photoinitiators
The photo polymerization has characteristic as well as free radical polymerization which the first step is photoinitiation. Photoinitiator is a catalyst used to photo polymerization with absorption of light to produce reactive species. In the photo polymerization there is propagation chain growth and termination chain growth (include chain transfer and disproportionation). Photo initiators have two types to generate reactive species. The first type is very reactive to break double bonds after getting photon. Thus, the second type form excited tripled state for hydrogen absorption and electron transfer. In this experiment we used the first type of photo initiators that is IRGACURE 2959 with using range of wavelengths of UV about 300-400 nm (the structure can be seen figure1).
According to photo polymerization is classified by three classifications those are photo initiation of free radical, ionic polymerization, topochemical polymerization (crystal-crystal transformation). In this experiment we classified in photo polymerization of free radical. Free radical species can be generated by five ways of photo initiations. They are:
1. Unimolecular fragmentation of type I photo initiators
2. Bimolecular reactions of type II photo initiators
3. Macromolecular photo initiators
4. Photo initiators for visible light is used to free radical polymerization, they are:
- a. Metal-based initiators
- b. Dye/co-initiator systems
- c. Quinones and 1,2-diketones
5. Inorganic photo initiators
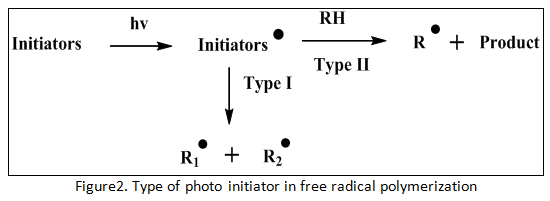
We knew detail about classification of photo initiators in above sentences that theoretically photo initiators of free radical polymerization have two types with different treatment in their reaction (see detail we can see figure 2).
Reaction Mechanism
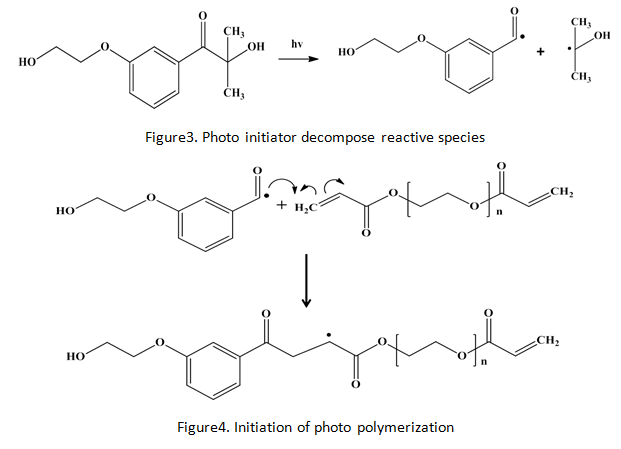
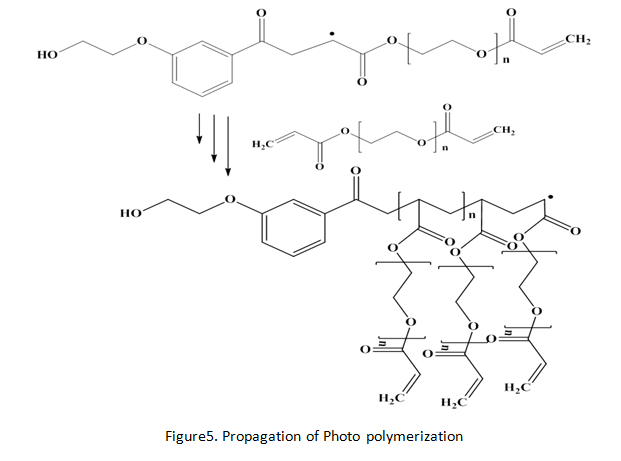
Experiment
In this experiment we have to mix between PEG 400-diacrylate and IRGACURE 2959 under stirring to get homogeneous mixture. In this experiment we drove mixture at high vacuum to remove gas bubbles generated. In this experiment we got two products with same process but different characteristics. As the first product irradiate the whole film and the second product irradiate not at all part of surface (using overlay).
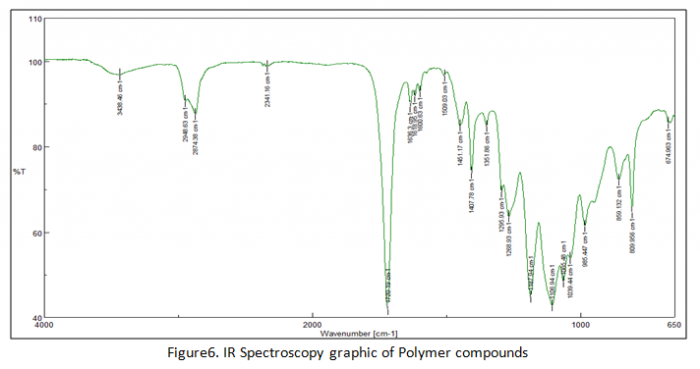
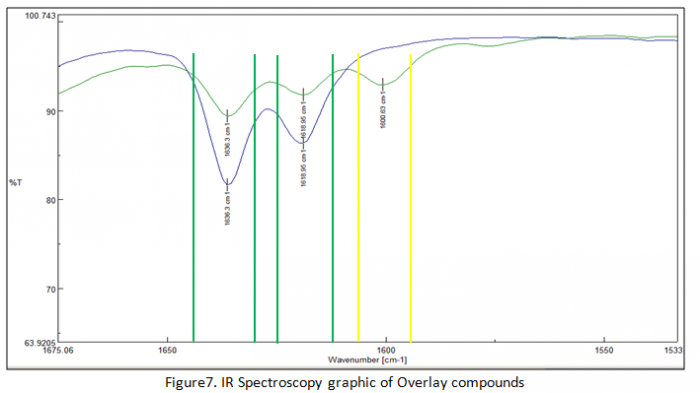
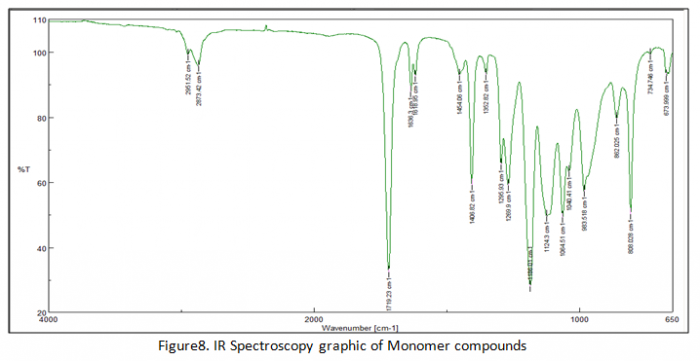
In the end of experiment we got result of IR spectroscopy analysis in figure 6, 7 and 8. In figure 6 that explain transmitter of polymers chain. Actually figure 6 and figure there are no really different compound in their structure but only at transmitter of every component. Figure 7 explains about overlay which in this figure there are two lines (blue and green line). Blue line has wavelength of chains at two areas which the first area has range 1648-1630 cm-1 and the second area has 1625-1613 cm-1. On the other hand green line has three areas for explaining about chain compounds in their structure.
As comparison between polymers and monomer graphic those are some different transmittance of each wavelength areas. Polymers has lower transmittance than monomer transmittance because polymers has long chains (combination of monomers) in order to the light can be absorb more than monomers. In this experiment we have to know about the formula of transmittance is:
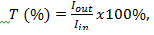
Baca konten-konten menarik Kompasiana langsung dari smartphone kamu. Follow channel WhatsApp Kompasiana sekarang di sini: https://whatsapp.com/channel/0029VaYjYaL4Spk7WflFYJ2H













